11 Dec Fixed-Dose Combination (FDC) Problem
This article covers “Daily Current Affairs” and the topic details “ Fixed Dose Combination (FDC) Problem”. This topic has relevance in the Social Issues section of the UPSC CSE exam.
GS 2: Social Issues
Why in the news?
A recent study conducted by academics from India, Qatar, and the United Kingdom and published in the Journal of Pharmaceutical Policy and Practice reveals concerning findings. The study underscores the prevalence of unapproved and, in some cases, banned fixed dose combinations (FDCs) of antibiotics being marketed in India.
Background:
The study, utilizing pharmaceutical industry sales data, unveils critical insights into the prevalence of unapproved and banned Fixed Dose Combinations (FDCs) of antibiotics in the year 2020.
Disturbing Statistics:
- Unapproved FDCs: A staggering 60.5% of antibiotics FDCs, comprising 239 formulations, were found to be unapproved based on the sales data.
- Banned FDCs: An additional 9.9%, constituting 39 formulations, were still being sold despite being officially banned in the country.
Concerns about Antibacterial Microbial Resistance (AMR):
The revelation that a significant proportion of these unapproved or banned FDCs includes antibiotics raises alarming concerns. This is particularly pertinent due to the escalating prevalence of antibacterial microbial resistance (AMR) in India.
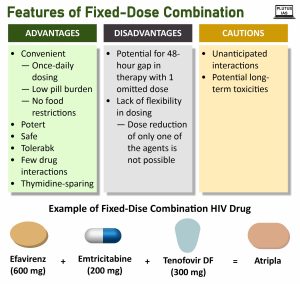
Understanding Fixed Dose Combination (FDC) Phenomenon:
- Definition: FDCs represent combinations of one or more established drugs, often employed to enhance patient compliance in the treatment of certain diseases.
- Rationale for FDCs: In scenarios where a patient requires multiple medications for a specific treatment, the use of FDCs can streamline the process and improve compliance. By consolidating multiple drugs into a single tablet or syrup, the likelihood of patients forgetting to take individual medications is reduced.
- Positive Example: Notably, diseases like AIDS have seen documented success in using FDCs, significantly enhancing patient compliance and, consequently, improving treatment outcomes.
Therapeutic Considerations:
- Therapeutic Efficacy: The interaction between different drugs within FDCs can impact their efficacy, potentially enhancing or inhibiting each other’s effects, leading to unpredictable outcomes.
- Toxicity Concerns: The formulation of FDCs carries the risk of creating metabolites or by-products that may be more toxic than the individual components. Rigorous evaluation during the formulation process is crucial.
- Excipient Interaction: Even though excipients are considered inactive, they can interact with active ingredients or among themselves, influencing the overall performance and safety of the formulation.
Regulatory Challenges:
- Unregulated FDCs: The issue of unregulated FDCs has been longstanding, with regulatory amendments granting power to the central government to prohibit drugs lacking therapeutic value. However, enforcement has been deficient, and the lack of prosecution for violations raises concerns about regulatory oversight.
- State-level Enforcement: State drug controllers’ failure to adhere to laws and issue manufacturing licenses for unapproved FDCs adds to regulatory challenges, highlighting the need for consistent enforcement.
Pharmaceutical Industry Dynamics:
- Regulatory Evasion and Pricing Strategies: The use of FDCs by the pharmaceutical industry to evade regulations, particularly the Drugs (Prices Control) Order (DPCO), is a significant concern. Combining drugs enables companies to bypass government-set price regulations for individual drugs, potentially impacting market competition and pricing.
- Lack of Standards: The diverse range of FDCs and the absence of clear testing standards contribute to challenges in ensuring the quality of these combinations. The lack of recognized standards allows manufacturers to establish their own testing protocols, raising questions about result reliability.
- Dubious FDC Combinations: The introduction of FDCs without a sound medical rationale, such as combining unrelated drugs, raises concerns about the safety and efficacy of these combinations, posing potential risks to patient health.
- Competition and Pseudo-Innovation: Creating FDCs to circumvent market competition for individual drugs can lead to pseudo-innovation. This strategy allows companies to claim uniqueness and charge higher prices until competitors introduce similar products, impacting market dynamics.
Emphasis on Rigorous Scientific Evaluation:
- Scientific Evaluation: The approval process for FDCs must undergo comprehensive scientific evaluation, encompassing preclinical studies and clinical trials explicitly designed to assess safety, efficacy, and potential interactions of combined drugs.
Establishment of Strict Regulatory Standards:
- Global Standards Adoption: Regulatory agencies globally adhere to stringent standards for FDC approvals. This involves not only evaluating individual drugs but also scrutinizing interactions, synergies, or antagonisms. Implementing comparable standards in India is imperative.
Implementation of Post-Market Surveillance:
- Continuous Monitoring: Beyond approval, the implementation of post-market surveillance is crucial. Real-world monitoring of FDC use helps identify unexpected side effects or interactions that may not have been apparent during the pre approval stages.
Prioritizing Patient Safety:
- Central Role in Approval: Patient safety should be the focal point throughout the approval process. Striking a balance between therapeutic innovation and patient well-being is essential for the responsible introduction of FDCs into the market.
Source: India’s alarming ‘fixed dose combination’ problem – The Hindu
Download plutus ias current affairs eng med 11th Dec 2023
Q.1 With reference to Fixed-Dose Combinations (FDCs):
- FDCs combine established drugs to enhance patient compliance.
- They streamline treatment by consolidating multiple drugs, reducing the chance of missed doses.
Which of the statements is/are correct?
(a) 1 only
(b) 2 only
(c) Both 1 and 2
(d) Neither 1 nor 2
ANSWER: C
Q.2 Discuss the challenges and opportunities associated with the concurrent issues of Antimicrobial Resistance (AMR) and the use of Fixed-Dose Combinations (FDCs) in the context of healthcare in India. Assess the strategies that could be adopted to address these issues and promote effective public health management.



No Comments