03 Aug “Biomarkers: Pioneering the Next Generation in Health Sector”
“Biomarkers: Pioneering the Next Generation in Health Sector”
This article covers “Daily Current Affairs” and details the topic of biomarkers; types, and applications.
Syllabus Mapping:
GS-3-Science and Technology-Biotechnology: Awareness in the fields of biotechnology
For Prelims:
What are the biomarkers and their various types?
For Mains:
What is the application of the biomarkers and their role in the health sector?
Why In News
Scientists may soon uncover more insights into the evolutionary significance of biomarker ERVs and their contribution to human biology. This will lead to breakthroughs in regenerative medicine, cancer therapies, and personalized medicine, enhancing our understanding of human health and evolution.
Source: TH, only for refenece purposes.
What are the Biomarkers?
Definition: Biomarkers, also known as biological markers, are quantifiable indicators of a biological state or condition. These can include molecules, genes, proteins, or other features within the body that reveal either normal or abnormal biological processes, or responses to treatment.
Types of Biomarkers
- Genetic Biomarkers: Examples: Specific DNA sequences or gene mutations, like BRCA1/BRCA2 for breast cancer risk.
- Protein Biomarkers: Examples: Enzymes, hormones, or proteins like PSA (Prostate-Specific Antigen) for prostate cancer.
- Metabolic Biomarkers:Examples: Metabolites like glucose or cholesterol levels.
- Imaging Biomarkers: Examples: Tumor size or brain activity patterns observed via MRI or PET scans.
Working of Biomarkers
- Detection: Biomarkers can be detected through various methods, including blood tests, imaging, and tissue biopsies.
- Measurement: Levels or presence of biomarkers are measured and compared to normal ranges or patterns associated with specific conditions.
- Interpretation: The detected levels are interpreted to understand the biological state or disease. For example, elevated levels of certain proteins can indicate inflammation or cancer.
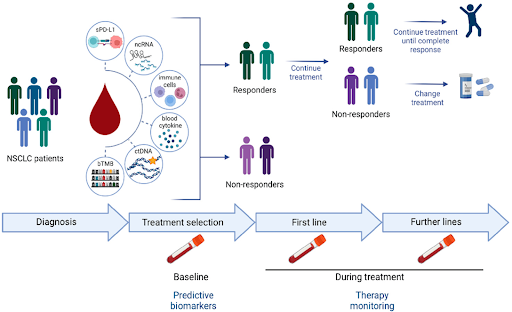
Fig: Working mechanism of blood-based biomarkers.
Source: MDPI only for reference purposes.
Recent Development:
The recent discoveries related to ERVs may lead to advancements in regenerative medicine, cancer therapies, and personalized medicine, enhancing our understanding of human biology and evolution.
Biomarkers Derived from Endogenous Retroviruses (ERVs)
Syncytins and Placental Development
- Syncytins: A class of genes derived from endogenous retroviruses (ERVs), which have become essential for placenta formation in mammals.
- Evolutionary Importance: Syncytins played a crucial role in the development of placentas, an adaptation critical for mammalian evolution from egg-laying ancestors.
- Recent Findings: Research from Sun Yat Sen University identified a specific RNA, derived from an ERV, that is dysregulated in early-onset preeclampsia. This RNA could potentially serve as a biomarker for diagnosing preeclampsia, a pregnancy complication characterized by elevated blood pressure.
Tumor Formation and Cell Differentiation
- MERVL-gag Protein: An ERV-derived protein that regulates the transition of cells from totipotency (ability to become any cell type) to pluripotency (ability to become specific cell types). MERVL-gag is crucial in controlling other proteins involved in this transition, and works with URI protein to facilitate the embryo’s developmental stages. These findings were published in Science Advances.
- LTR10 Retroelement: A human ERV element integrated into the genome approximately 30 million years ago. A study from the University of Colorado, Boulder, found that LTR10 influences tumor formation in colorectal cancer. The retroelement’s presence is linked to large genomic changes and can affect gene expression related to tumor development.
Examples of Biomarkers in Use
- Cancer:
- CA-125: Biomarker for ovarian cancer.
- HER2/neu: Protein overexpression in breast cancer.
- Cardiovascular Disease:
- Troponin: Protein released during a heart attack, indicating myocardial injury.
- B-type Natriuretic Peptide (BNP): Elevated in heart failure.
- Neurodegenerative Diseases:
- Amyloid-Beta: Protein associated with Alzheimer’s disease, detectable in cerebrospinal fluid or PET scans.
- Infectious Diseases:
- Viral Load: Measures the amount of virus in the blood, used in monitoring HIV treatment.
Applications of Biomarkers
Disease Diagnosis:
Early Detection: Biomarkers can help in the early detection of diseases, such as cancer, before symptoms appear. For example, elevated PSA levels can suggest prostate cancer.
Confirmation:Used to confirm a diagnosis when symptoms are ambiguous.
Disease Prognosis:
Predicting Outcomes: Biomarkers can predict the progression or outcome of a disease. For example, certain gene expressions might indicate aggressive forms of cancer.
Treatment Monitoring:
Response Assessment: Used to monitor how well a treatment is working. For example, tracking changes in tumor markers to assess chemotherapy efficacy.
Adjusting Therapy: Helps in adjusting treatment plans based on the biomarker levels, improving personalized medicine.
Personalized Medicine:
Tailoring Treatments: Biomarkers enable personalized treatment strategies based on individual genetic or molecular profiles. For instance, targeted therapies in cancer treatment are based on specific genetic mutations.
Drug Development:
Target Identification: Biomarkers are used to identify potential drug targets and to validate the effectiveness of new drugs during clinical trials.
Safety Monitoring: Assess potential side effects of new drugs by monitoring changes in biomarker levels.
Public Health:
Screening Programs: Utilized in large-scale screening programs for diseases like diabetes, cancer, or cardiovascular diseases to identify at-risk populations.
Issues and challenges in the use of Biomarkers:
- Standardization: Variations in testing procedures for biomarkers can lead to inconsistent results and undermine the utility of biomarkers.
- Interpretation: The interpretation of biomarker levels can be complex. A biomarker might be elevated due to a range of factors other than the disease in question, leading to potential false positives or negatives.
- Ethical and Privacy Concerns: The use of biomarkers, especially genetic ones, raises ethical issues related to privacy, consent, and potential misuse of sensitive information. Concerns about how this data is stored, shared, and used need to be carefully managed.
- Cost and Accessibility: Advanced biomarker testing can be expensive and may not be accessible to all patients, leading to disparities in healthcare. Ensuring that biomarkers provide value relative to their cost is crucial.
- Biological Variability: Individual differences such as age, sex, genetics, and lifestyle can affect biomarker levels, making it challenging to establish universal thresholds and cut-offs.
- Regulatory and Approval Processes: Navigating the regulatory pathways for biomarker tests can be complex and time-consuming. Biomarkers need to meet specific regulatory requirements before they can be widely used in clinical practice.
- Dynamic Nature of Biomarkers: Some biomarkers may change over time or in response to treatment, requiring ongoing monitoring and adjustment of interpretation.
- Potential for Overreliance: There’s a risk that clinicians might rely too heavily on biomarkers for diagnosis or treatment decisions, potentially overlooking other important aspects of patient care.
- Biomarker Discovery and Development: Identifying new biomarkers and developing tests for them involves significant research and development, which can be a lengthy and costly process.
Potential ways to tackle the challenges related to the biomarkers:
- Enhanced Biomarker Validation: Conduct extensive multi-center studies to validate biomarkers across diverse populations and conditions, and develop standardized guidelines for their validation and application.
- Unified Testing Standards: Establish and enforce unified testing protocols and calibration methods across laboratories, and implement regular quality control checks and inter-laboratory comparisons to ensure consistent results.
- Integrated Diagnostic Approach: Use biomarkers alongside clinical assessments and patient history for more accurate diagnostics, and develop advanced bioinformatics tools to assist in interpreting biomarker data.
- Ethical Data Management: Ensure patients are fully informed about the use and storage of their biomarker data, and implement strong data protection measures to safeguard patient privacy in compliance with regulations.
- Cost-Effective Access Solutions: Evaluate the cost-effectiveness of biomarker tests and explore ways to reduce costs through technological advancements while developing programs to improve access to testing in underserved areas.
- Personalized Interpretation Models: Tailor biomarker interpretation to individual patient profiles, considering genetics and lifestyle, and use dynamic models that account for biological variability and adjust over time.
- Streamlined Regulatory Approvals: Advocate for more efficient regulatory pathways that balance thoroughness with timely introduction of new biomarkers, and engage early with regulatory bodies to ensure compliance.
- Longitudinal Monitoring Strategies: Conduct longitudinal studies to understand how biomarkers change over time and in response to treatments, and develop adaptive testing strategies that adjust based on ongoing biomarker changes.
- Comprehensive Clinical Integration: Promote a holistic approach that integrates biomarkers with other clinical data, and provide education and training for clinicians on the appropriate use of biomarkers and comprehensive patient evaluations.
- Collaborative Discovery and Innovation: Encourage collaboration between academic institutions, industry, and healthcare providers to accelerate biomarker discovery and development, and increase funding and investment to support research and innovative testing methods.
Conclusion:
Biomarkers hold immense potential across various sectors of healthcare, offering improved diagnostics, personalized treatments, and advancements in drug development. By addressing the challenges through rigorous validation, standardization, ethical practices, and comprehensive strategies, the full benefits of biomarkers can be realized. Ensuring that biomarkers are reliable, accessible, and integrated into a holistic healthcare approach will lead to more accurate diagnoses, better treatment outcomes, and overall enhancements in patient care. With continued research, collaboration, and innovation, biomarkers can significantly transform and advance modern medicine.
Download plutus ias current affairs eng med 03rd Aug 2024
Prelims Question:
Q. In which of the following are biomarkers used?
- Diagnosis of prostate cancer in patients.
- Measurement of the virus load in the blood of humans.
- The early prediction of preeclampsia.
Select the correct answer using the code given below:
(a) 1 only
(b) 2 and 3 only
(c) 1 and 3 only
(d) 1, 2 and 3
Answer: d.
Mains Question:
“Biomarkers have the potential to revolutionize the health sector but there is a need to balance the various challenges associated with it and its use in various fields”. Comment?
(150 words 10 marks)



No Comments