10 Oct WHO put a ban on Indian Cough Syrup
WHO put a ban on Indian Cough Syrup
The topic is based on The WHO putting a ban on Indian Cough Syrup. The article talks about The Economic and Social Development of India’s significance of The ban on Indian Cough Syrup by the WHO.
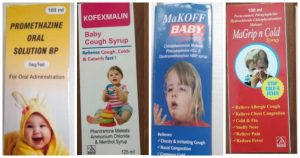
Pic: The ban on Indian Cough Syrup by the WHO
Prelims: national and international current affairs
Mains: GS II: Important International institutions, agencies, and fora- their structure, mandate
Story behind
- The World Health Organization (WHO) has raised an alert over an Indian company that made four fevers, cold and cough syrup after 66 children died in the Gambia, an African Country, urging people to not use them.
What did the WHO say?
- “The four medicines are cough and cold syrups produced by Maiden Pharmaceuticals Limited, in India. They failed the test as they have unacceptable amounts of diethylene glycol and ethylene glycol as contaminants”, the global health agency stated. According to the tentative results received by the WHO, four out of the 23 samples tested have been found to contain either Diethylene Glycol/Ethylene Glycol.
- All four syrups — Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup, and Magrip N Cold Syrup – are made by Haryana-based Maiden Pharmaceuticals.
About World Health Organization (WHO)
- It is a particularized agency of the United Nations responsible for international public health
- The Headquartered Of WHO is in Geneva, Switzerland, it has six regional offices and 150 field offices worldwide
- It was established on 7 April 1948
- The WHO Constitution states its main objective as “the attainment by all peoples of the highest possible level of health”
- Laboratory analysis of samples of each of the four products confirms that they contain unacceptable amounts of diethylene glycol and ethylene glycol as contaminants.
The objectives of the WHO are
- To operate as the directing and coordinating authority on international health work
- To setup and support effective collaboration with the United Nations, specialized agencies, governmental health administrations, professional groups, and such other organizations as may be deemed appropriate
- To facilitate Governments, upon request, in strengthening health services
- To furnish appropriate technical assistance and, in emergencies, necessary aid upon the request or acceptance of Governments
- To supply or assist in providing, upon the request of the United Nations, health services and facilities to special groups, such as the peoples of trust territories
- To establish and maintain such administrative and technical services as may be required, including epidemiological and statistical services
- To boost and advance work to eradicate epidemic, endemic, and other diseases
- To encourage, in cooperation with other specialized agencies where necessary, the prevention of accidental injuries
- To encourage, in cooperation with other specialized agencies where necessary, the improvement of nutrition, housing, sanitation, recreation, economic or working conditions, and other aspects of environmental hygiene
- To help in cooperation among scientific and professional groups which contribute to the advancement of health
- To develop conventions, agreements and regulations, make recommendations with respect to international health matters, and perform
WHO and India
- India became a party of WHO on 12 January 1948.
- The regional office for South East Asia is located in New Delhi.
- The first session of the WHO regional committee was held in 1948, and was inaugurated by Pandit Jawaharlal Nehru, Prime Minister of India, and was addressed by the WHO Director-General
Di-ethylene Glycol and Ethylene Glycol
- Di-ethylene Glycol and Ethylene Glycol are considered venomous for the human body. They can be the cause of kidney and neurological toxicity when consumed. This toxicity has been found a cause in several cases of mass poisoning when consumed via drugs.
- They are Hygroscopic sweetish liquid that has no color and no particular smell. They can make a homogenous mixture of water, alcohol, ether, acetone, and ethylene glycol.
- The poisonous effects of Diethylene Glycol and Ethylene Glycol can result in abdominal pain, vomiting, diarrhea, inability to pass urine, headache, altered mental state, and acute kidney injury.
- As per a paper in the National Library of Medicine, 10 DEG mass poisonings have occurred over the past 70 years. These mass poisonings were all caused by DEG-contaminated liquid or ointment medications. DEG contamination happens when it is used in medicinal products instead of safer—but more expensive—diluents such as pharmaceutical-grade glycerin.
Difference between Di-ethylene Glycol and Ethylene Glycol
- Ethylene glycol is commonly used in the manufacturing of polyester fibers, paints and polyethylene terephthalate (PET)
- On the other hand, DEG is used to break fluid in cigarette and paper treatment and also in some dyes, due to its hygroscopic property,
How these contaminants enter the drug-supply chain
- Complexities in the distribution of glycerin and other pharmaceutical raw materials that may involve many import and export handlers cause these contaminants to creep into such glycerin-based medical products.
- DEG has also been used illegally as a cheap substitute solvent in drug manufacturing.
- As a result of it, it contributes to the risk of such adulteration in inadequate international regulations and the lack of analytical methods, according to scientists.
Symptoms
- The adulterant can result in renal and neurological toxicity if ingested, which, in common language, they can affect the brain and kidney functions.
- Acute kidney injury is commonly the main cause of death, which occurs anywhere between 8 and 24 hours after exposure if the dose is high.
Central Drugs Standard Control Organization CDSCO
- The CDSCO stands for Central Drugs Standard Control Organization (CDSCO)
- It is the national regulatory body of India, regulated the following :
- Cosmetics
- Pharmaceuticals
- Medical devices
- It also executes an alike job to the:
- European Union’s European Medicines Agency
- Japan’s PMDA
- The United States Food and Drug Administration (FDA)
- The United Kingdom’s Medicines and Healthcare Products Regulatory Agency
- China’s National Medical Products Administration (NMPA)
- The Government of India has announced a proposal to have the CDSCO assess all medical devices, including implants and contraception.
- The Drug Controller General of India (DCGI) regulates pharmaceuticals and medical devices under the CDSCO.
- The body works under the Ministry of Health and Family Welfare.
- The Drug Technical Advisory Board (DTAB) and the Drug Consultative Committee (DCC) advise the DCGI.
Some of the roles of CDSCO are as follows
- Specify the standards and measures for ensuring the safety, efficacy, and quality of drugs, cosmetics, diagnostics and devices in the country.
- Supervises the market authorization of new drugs and clinical trial standards.
- Govern the drug imports and approves licenses to manufacture the above-mentioned products.
- It also controls the export of drugs in India, any manufacturer with certification from CDSCO can export drugs outside India.
The Related Regulations in India
- The Drugs and Cosmetics Act:
- The Drugs and Cosmetics Act, of 1940 and Rules 1945 have assigned various responsibilities to central and state regulators for the regulation of drugs and cosmetics.
- It stipulates the administrative guidelines for issuing licenses to manufacture Ayurvedic, Siddha, and Unani medicines.
- It is compulsory for the manufacturers to practice according to the prescribed requirements for licensing of manufacturing units & medicines along with proof of safety & effectiveness, and compliance with the Good Manufacturing Practices (GMP).
Sources:
Daily Current Affairs for UPSC
Current Affairs is an important factor of any competitive examination like the Government Job exam for example UPSC, IAS, SSC, NET, and other exams. Reading daily current affairs helps you to increase your general knowledge. You get new ideas by reading new current affairs daily. If you are looking for daily current affairs for your UPSC exam preparation, Plutus IAS will provide the best daily current affairs free of cost. So get the top 10 current affairs from us and read current affairs every day.
Download PDF now :



No Comments